Abstract
Introduction Primary central nervous system lymphoma (PCNSL) is an aggressive non-Hodgkin lymphoma (NHL) which is confined to the central nervous system at diagnosis. Current standards imaging modality for PCNSL is T1-weighted (T1W) contrast-enhanced magnetic resonance imaging (MRI). However, brain MRI has some limitations of assessing the initial tumor burden or mid-treatment response to predict the clinical outcome. There are limited data exploring the value of 18F-FDG brain PET/CT in PCNSL. Therefore, this study analyzed the prognostic significance of interim 18F-FDG brain PET/CT response assessment in patients with PCNCL.
Patients and methods Fifty-six patients with PCNSL between January 2016 and July 2021 were screened for this prospective cohort study. Among the 56 patients, 3 patients did not have baseline 18F-FDG brain PET/CT scan. Among the remaining 53 patients, 9 patients who had negative finding on their initial PET/CT were excluded and 44 patients who had positive standardized uptake value (SUV) uptakes on lesions were finally included. Induction chemotherapy consisted of HD-MTX (3.5g/m2) on day 1 and cytarabine (2.0g/m2) on days 2 and 3 with or without rituximab (375mg/m2) on day 1 every 3 weeks for transplant eligible patients and HD-MTX (3.5g/m2) alone with or without rituximab (375mg/m2) every 2 weeks for elderly patients, respectively. Interim 18F-FDG brain PET/CT was tested after induction therapy, and patients who achieved more than PR after induction treatment proceeded to consolidation treatment consisted of etoposide (375mg/m2) and cytarabine (3g/m2) on days 1 and 2. The clinical prognostic factors were assessed according to International Extranodal Lymphoma Study Group (IESLG) guideline. Tumor to normal tissue ratio (T/N ratio) on 18F-FDG brain was estimated as the ratio of the SUVmax of the brain lesion to the SUVmax of contralateral normal frontal gray matter. If the patients had multiple lesions, the lesion showing the highest SUVmax was evaluated for PET response.
Results All of the patients had been diagnosed with diffuse large B-cell lymphoma. According to the IELSG risk score, 11 patients (25.0%) were high risk, 29 patients (65.9%) were intermediate risk, and 4 patients (9.1%) were low risk. Among 44 patients, 33 patients (75.0%) showed more than partial response (PR) after induction treatment, and 12 patients (36.4%) proceeded to autologous stem cell transplantation (ASCT).
Among the 44 patients, 3 patients experienced disease progression before assessing interim 18F-FDG PET/CT and these patients were excluded from interim response assessment and survival analysis. Twenty-four patients achieved complete molecular response (CMR), among whom 1 complete response (CR), 2 unconfirmed CR (CRu) and 21 PR were observed based on early brain MRI response assessment. Among 17 patients who did not achieved CMR by interim PET, 8 PR and 9 stable disease (SD)/progressive disease (PD) were assessed by early brain MRI assessment. In 29 patients with MRI-assessed PR, PET was found positive and negative in 8 and 21 cases.
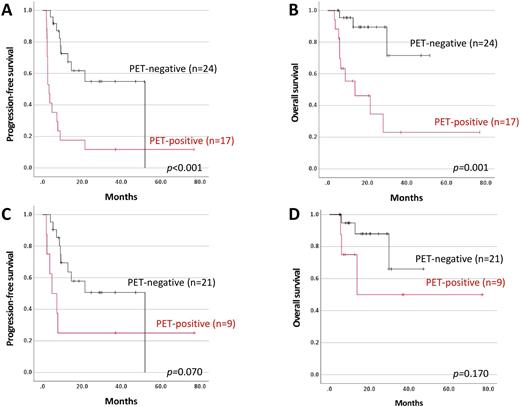
Patients who achieved CMR based on interim PET assessment showed significantly longer PFS than the patients who did not achieved CMR (52.0 months vs. 3.3 months, p<0.001, Figure 1A). OS appeared superior in the patients with CMR in interim PET assessment than in the patients who did not (Not reached vs. 13.8 months, p=0.001, Figure 1B). In 29 patients who were assessed as PR by interim brain MRI, the patients with PET-negative showed longer PFS than the patients with PET-positive although the difference was not reached statistical significance (52.0 months vs. 4.7 months, p=0.07, Figure 1C). OS tended to be longer in the patients with PET-negative (Not reached vs. 13.8 months, p=0.170, Figure 1D).
Conclusion Achievement of CMR in interim 18F-FDG brain PET/CT has a prognostic significance and may provide additional clinical information of predicting survival outcome in PCNSL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal